StudentShare


Our website is a unique platform where students can share their papers in a matter of giving an example of the work to be done. If you find papers
matching your topic, you may use them only as an example of work. This is 100% legal. You may not submit downloaded papers as your own, that is cheating. Also you
should remember, that this work was alredy submitted once by a student who originally wrote it.
Login
Create an Account
The service is 100% legal
- Home
- Free Samples
- Premium Essays
- Editing Services
- Extra Tools
- Essay Writing Help
- About Us
✕
- Studentshare
- Subjects
- Engineering and Construction
- The Best Metal Which Is Most Corrosion-Resistant
Free
The Best Metal Which Is Most Corrosion-Resistant - Lab Report Example
Summary
The paper "The Best Metal Which Is Most Corrosion-Resistant" states that paint is one way of preventing oxidation and corrosion, and this is because paints prevent oxidation to take place and also prevent electrons from moving from one point to another. …
Download full paper File format: .doc, available for editing
GRAB THE BEST PAPER98.7% of users find it useful
- Subject: Engineering and Construction
- Type: Lab Report
- Level: Masters
- Pages: 5 (1250 words)
- Downloads: 0
- Author: gisselle33
Extract of sample "The Best Metal Which Is Most Corrosion-Resistant"
Mechanical Engineering W5446, Selection of Common Engineering Materials Document type: Essay Area: Mechanical Engineering Date: 18th March, 2015
Introduction
The main aim of this experiment was to determine the best metal which is most corrosion-resistant, to be used in buildings dogs’ enclosure
Definition of terms
Rust is the reddish matter that’s forms on the surface of the metal when they come into contact with air or moisture while, corrosion is a gradual destruction of metal due to the chemical reaction. The list of metal that was investigated were; copper, iron, steel, stainless steel, aluminum, and zinc.
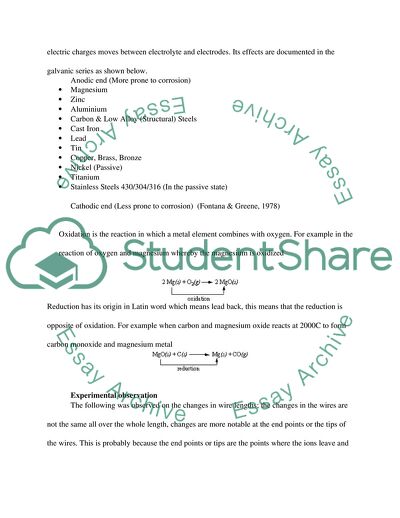
Electrochemistry is one branch of physical chemistry that’s concentrates on the chemical reaction that takes place in an electrode, electrolyte, and ionic conductor’s interfaces, whereby electric charges moves between electrolyte and electrodes. Its effects are documented in the galvanic series as shown below.
Anodic end (More prone to corrosion)
Magnesium
Zinc
Aluminium
Carbon & Low Alloy (Structural) Steels
Cast Iron
Lead
Tin
Copper, Brass, Bronze
Nickel (Passive)
Titanium
Stainless Steels 430/304/316 (In the passive state)
Cathodic end (Less prone to corrosion) (Fontana & Greene, 1978)
Oxidation is the reaction in which a metal element combines with oxygen. For example in the reaction of oxygen and magnesium whereby the magnesium is oxidized
Reduction has its origin in Latin word which means lead back, this means that the reduction is opposite of oxidation. For example when carbon and magnesium oxide reacts at 2000C to form carbon monoxide and magnesium metal
Experimental observation
The following was observed on the changes in wire lengths; the changes in the wires are not the same all over the whole length, changes are more notable at the end points or the tips of the wires. This is probably because the end points or tips are the points where the ions leave and enter the wires. So there is either corrosion or deposition of materials, depending on the position of the concerned metal in the galvanic series
When the three (3) sets of wire are compared, while using the galvanic series, metals that are Topmost on list are corroded more than metal below the list during galvanic corrosion.
For the set of wires kept in tap water, the rate of corrosion decrease from zinc to stainless steel in this order; Zinc, Aluminum, Cast Iron, Copper, Stainless Steels.This mean, zinc is corroded most while stainless steels are corroded list
For the set of wires kept in salt water, the effects of corrosion is more as compared to in tap water, their order is as follows; Zinc, Aluminum, Cast Iron, Copper, Stainless Steels .This means that zinc is most affected while stainless steels are less affected
For the set of wires kept in air, the effects on metal are due oxidation and this oxidation affects metals that have ferrous in it or metals that are magnetic. Therefore, the order of oxidation is as follows; Cast Iron, Stainless Steels, Aluminum, Copper, Zinc. This because Copper and Zinc does not have ferrous as parts of its components. The cast-iron is most affected while copper and zinc are least affected by rust.
The following is the grade rating that describes the changes in the observation
Scale
5
4
3
2
1
Material
zinc
aluminum
Cast iron
copper
Stainless steel
Changes in Salt water
Scale
1
2
3
4
5
Material
zinc
aluminum
Cast iron
copper
Stainless steel
Changes in Tap water
Scale
5
4
3
2
1
Material
Cast iron
Stainless steel
aluminum
copper
Zinc
Changes in Fresh air
KEY: 5 ------- cause severe effect
3--------Moderate effect
2--------Least effect
The following are graphs drawn using above ratings and shows what happened to different metal under the three conditions
Conclusion
The combination of metals and environmental condition, which shows the greatest amount of oxidation are; Zinc in both salt and tap water and
Cast iron in fresh air
The combination of metals and environmental condition, which shows the least amount of oxidation are, Stainless steel in salt and Tap water,
Zinc and copper in fresh air
Variations
The following was observed on the changes of the weights of wires in different conditions; the weights will change the metals that were submerged in both salt water and tap water while the change in the metals that were place in the air is very minimal. Probably there would be a big change in weights in wires submerged in salt water as compared to wires submerged in tap water. This is because both corrosion and oxidation or reduction is higher in metals place in salty water. The weights change is due to deposition or corrosion on the metal surface while, in the air there is very minimum change because there are no electrons due water which can cause both oxidation and corrosion.
Temperature and salt concentration accelerates the rate of oxidation; this is because the temperature has two effects on the rate of reaction, first is the speed of reaction while the other one is due to the activation energy. This indicates that if there is limited energy to push the reaction then nothing will happen; also oxidation will double with every 10°C increase in temperature. Addition of more salt will increase the speed of oxidation, this is because there will be more ions in water so it contacts electricity better hence the rate of oxidation will go up.
When there is very little supply of oxygen in water, then the rate of oxidation goes down. This means that the wire will rust slowly. The water that is not boiled means there is allot of oxygen hence oxidation is very high. This means that the wire in jar with water that was not boiled will rust fast as compared to wire in jar with boiled water.
The change of PH of water affects the number of ions in the water, and these ions are responsible for conductivity. This means that when vinegar is added will make the water corrode the metals faster while addition of baking soda will make the water corrosive but not as corrosive as when it is acidic. This is because acidic water has Avery high rate of oxidation because of positive hydrogen ions (H+)
What happened to Titanic shipwrecks is what is called electrochemical corrosion or single metal corrosion which happens because the materials or iron cannonballs is made of alloy, this means that one metal in an alloy become a node while another one became cathode while the sea water becomes electrolyte and the alloy are in contact with one another creating path for the flow of electron and causes corrosion. This process is not very serious and does not cause a major concern in the shipping industry. When this iron cannonballs is brought to the surface from the shipwreck location, the reaction or corrosion will stop because there are no materials that can act as electrolyte to enable the movements of ions. A good experiment to do in order to learn more about this phenomenon is too deep or submerge one wire which is an alloy in individual jar contain salt water and tap water. The changes in the wires should be noted for a period of one month while in the water. It is then exposed to fresh air and monitored for a period of one month. It will be noted that there is a corrosion taking place in wires submerged in water, but it is more pronounced in salt water, it will also be noted that the there will be no more corrosion taking place when the wires are taken out of water and exposed to fresh air .
Paint is one way of preventing oxidation and corrosion, and this is because paints prevent oxidation to take place and also prevent electrons from moving from one point to another. Therefore, oxidation and corrosion happens on unpainted wires while it doesn’t occur in painted ones
Reference
Fontana, M.G, & Greene, N. D (1978).Corrosion Engineering (2nd ed., p. 32). New York: McGraw-Hill.
Read
More
sponsored ads
Save Your Time for More Important Things
Let us write or edit the lab report on your topic
"The Best Metal Which Is Most Corrosion-Resistant"
with a personal 20% discount.
GRAB THE BEST PAPER

✕
- TERMS & CONDITIONS
- PRIVACY POLICY
- COOKIES POLICY